LSD has staying power- chemistry explains why
But first, let’s clean up the name because, although LSD is called “acid,” it really shouldn’t be, at least according to chemists.
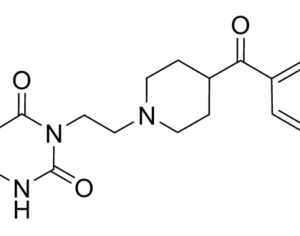
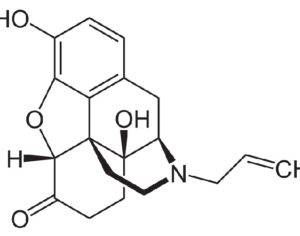
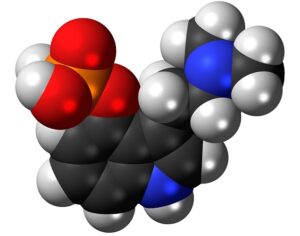
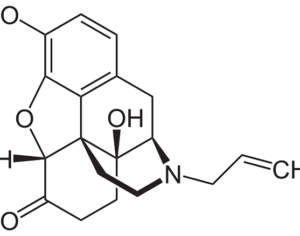
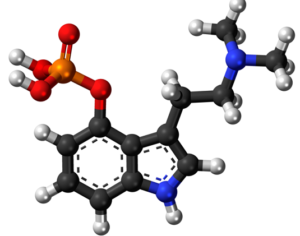
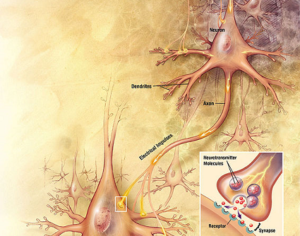
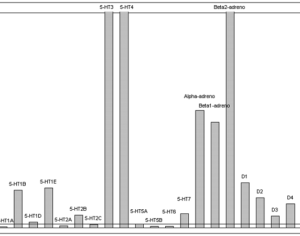
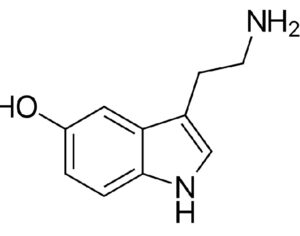
Lysergic acid, a natural product which is produced by ergot fungus, has, as the name implies, a functional group called a carboxylic acid (blue circle), hence the name. But lysergic acid is not LSD. LSD is not a natural chemical; instead is synthesized in a lab from lysergic acid. The correct name for LSD is LySergic acid Diethylamide. But the term “dropping amide” just doesn’t sound right, so users took some psychedelic license. Bryan Roth, M.D, Ph. D., and colleagues recently published a paper in the journal Cell entitled Crystal Structure of an LSD-Bound Human Serotonin Receptor. The paper contains some very elegant science, in particular, the details of how LSD binds to specific serotonin receptors. The details of this binding can be visualized at the atomic level by using a technique called x-ray crystallography, a very powerful tool for examining the chemical structures of molecules and how they interact with targets.
Original Article (ACSH):
LSD Has Staying Power- Chemistry Explains Why
Artwork Fair Use: Harshberger, John W.