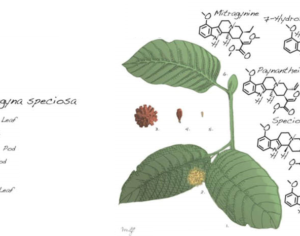
Molecular biologist disproves FDA kratom attacks
The FDA has clearly intentionally mischaracterized kratom using these unverified reports as the basis to recommend the effective ban on consumer access to kratom by placing kratom in Schedule I of the CSA. -Jane Babin, Ph.D., Esq. University of San Diego School of Law, J.D. Purdue University, Ph.D., Molecular Biology
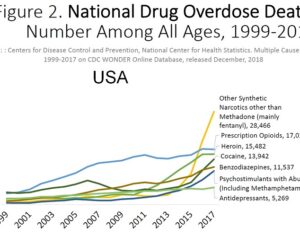
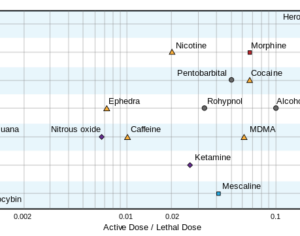
The FDA recommendation for the scheduling of kratom is supported only by exaggerated claims, discredited research, and distorted data, that in total fail to meet the evidentiary standard for placing kratom as a Schedule I controlled substance … The FDA publicly argues its transparent campaign to require kratom products to be subject to FDA authority in the submission of a new drug application. The FDA’s own data, however, fails to meet the criteria for CSA scheduling under Schedule I or any other schedule. Kratom remains a safe and suitable natural botanical for consumer use for products manufactured from the plant materials, including those crushed, chopped, powdered, or encapsulated … The DEA should decline further consideration of the FDA recommendation for the scheduling of kratom, and return it to the FDA with the direction that future recommendations must meet the same rigorous standards it requires for research prior to making any substantive public policy decision.
Original Article (American Kratom Association and Reddit):
Molecular biologist disproves FDA kratom attacks & Molecular biologist view
Artwork Fair Use: Andrew Dunn