What you need to know about kratom as the feds crack down on the herbal supplement
The first reports of pharmacological studies on mitragynine appeared in the literature in 1972 (Macko et al., 1972). Researchers at Smith, Kline, and French (SKF) were interested in finding a novel analgesic that would have less liability than the currently utilized opioids (i,e. morphine).
Their studies were the most comprehensive at the time and still remain as one of the more complete in the literature. A battery of animal studies were undertaken to investigate the analgesic potential and opiod actions of mitragynine. These studies did show that mitragynine had analgesic and antitussive properties comparable to codeine. Unlike codeine, mitragynine did not produce emesis [an act of instance of vomiting] or dyspnea [difficult or laboured breathing], was not blocked by nalorphine and had much less respiratory depression. Interestingly, it could also suppress the opioid withdrawal syndrome. Moreover, it was noted that mitragynine was active only via the oral and intraperitoneal [IP injection] routes of administration (in an equal ration), and was inactive via the subcutaneous route. It was hypothesized that the analgesic activity may be related to a metabolite, or that the bioavailability of mitragynine is influenced by the acidic conditions of the route administration. It appeared that Smith, Kline, and French (SKF) decided to abandon further studies of this substance, most likely due to the weak analgesic potency when compared to traditional, marketed opioid pharmaceuticals. -Christopher R. McCurdy, PhD, BS Ph, FAAPS (Department of Medicinal Chemistry, College of Pharmacy, University of Florida, Gainesville, Florida)
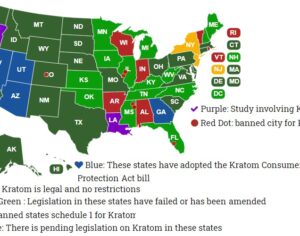
Why the Feds don’t like it. The FDA has repeatedly warned that kratom offers no medical benefits. The feds are cracking down. This week the FDA sent warning letters to three companies for marketing and distributing kratom as a treatment for opioid addiction, pain treatment and other symptoms, Marketwatch reported. “Despite our warnings that no kratom product is safe, we continue to find companies selling kratom and doing so with deceptive medical claims for which there’s no reliable scientific proof to support their use,” FDA Commissioner Scott Gottlieb said in a statement.
Why Fans like it. Kratom users say it quells pain, reduces anxiety, boosts mood and increases energy. Stories from users on the American Kratom Association website include testimonials from people with fibromyalgia, back pain, PTSD, arthritis, carpal tunnel syndrome, debilitating headaches and depression who have used it instead of prescription drugs. Susan Ash, the woman who founded the Colorado-based association, told Mother Jones how she became addicted to painkillers and a powerful opioid called Opana ER to deal with pain from Lyme disease eight years ago. In 2014 when she was trying to kick the opioid addiction, a woman in an online Lyme disease support group told her about kratom. She began taking it every day. In “literally two weeks, I became a productive member of society,” she told Mother Jones.
Original Article (Kansascity.com):
What you need to know about kratom as the feds crack down on the herbal supplement
Artwork/word Fair Use: By ECfES.org Public Lending Library : Ethnopharmacologic search for psychoactive drugs: 50th anniversary symposium, June 6- 8, 2017 Ghillean Prance-Dennis McKenna-Ben Loenen-Wade Davis – Synergetic Press, in association with Heffter Research Institute – 2018