Freedom of Information Request: FDA: evidence of claims in Kratom press release
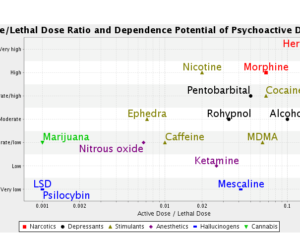
Here, I am also seeking to answer, with specificity, which opioids kratom resembles in terms of risk of death. Here, as “similar risk” has been mentioned, I am seeking the documentation and statistical analysis, used by the FDA, that articulates or provides evidence of the “similar risk” of kratom viz a viz other opioids. Additionally, I am also seeking any emails (whether sent to the FDA or any employee or director of the FDA, by a lobbying group, PAC, or any other outside source, as well as internally composed/generated emails). I additionally seek internal documents, including but not limited to memorandum of interviews and white papers, that offer the opinions or facts used to support the preceding, quoted, claims found in the press release. -Anthony Roberts, requestee (Tracking #2017-9756 Submitted Nov. 14, 2017 Due Dec. 13, 2017)
This is in response to your November 17, 2017, Freedom of Information Act (FOIA) request for records pertaining to “ALL EVIDENCE AND DOCUMENTATION RELIED UPON IN THE DRAFTING AND PUBLICATION OF THE AFOREMENTIONED PRESS RELEASE, TO SUPPORT THE FOLLOWING STATEMENTS [about Kratom], MADE BY THE FDA [here].” The Center Food Safety and Applied Nutrition (CFSAN) conducted a search and did not locate any records responsive to your request. Please be advised that your request has been submitted to one or more component offices within FDA. These offices will reply to you directly. CFSAN considers this request closed”. -CFSAN (The FDA is CFSAN’s Parent Agency)
Original Article (Muckrock):
Freedom of Information Request: FDA: evidence of claims in Kratom press release
Artwork Fair Use: Public Domain