Psychedelic biotech MAPS PBC rebrands to Lykos following FDA filing, scores $100M financing
MAPS PBC [Public Benefit Corporation) has rebranded to Lykos Therapeutics [a for-profit public benefit corporation (“PBC”)], (“Lykos”), the greek word for wolf.
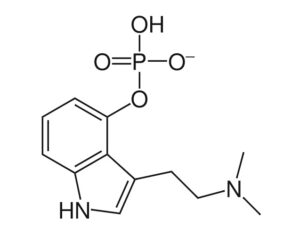
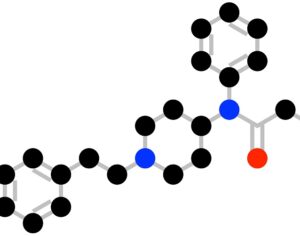
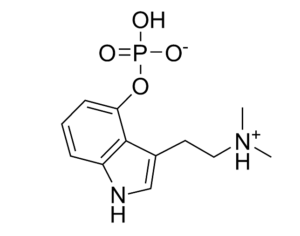
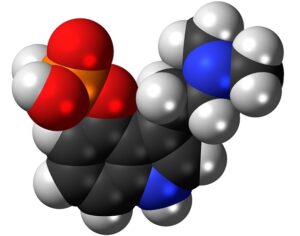
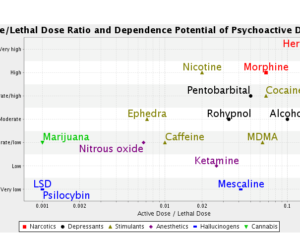
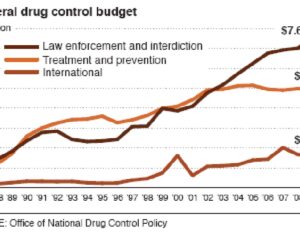
MAPS PBC spun out of its parent organization MAPS (Multidisciplinary Association for Psychedelic Studies)… clinical development effort of MDMA-assisted therapy [midomafetamine capsules used in combination with psychological intervention], which includes talk therapy, and other supportive services provided by a qualified healthcare provider… for post-traumatic stress disorder (PTSD)… submitted… new drug application to the FDA last month [December 2023]… ask[ing] regulators to approve a psychedelic as a therapeutic product. With Breakthrough Therapy designation given to MDMA in 2017… has requested the FDA grant Priority Review of the NDA [New Drug Application]. The FDA has 60 days to determine whether the NDA will be accepted for review and whether it will be a priority or standard review (six months or ten months, respectively). If approved by the FDA, the U.S. Drug Enforcement Administration (“DEA”) would be required to reschedule MDMA making it available for prescription medical use.
Original Article (Fierce Biotech & Lykos):
Psychedelic biotech MAPS PBC rebrands to Lykos following FDA filing, scores $100M financing & MAPS Public Benefit Corporation announces oversubscribed series a financing and renames to Lykos Therapeutics
Artwork Fair Use: Public Domain







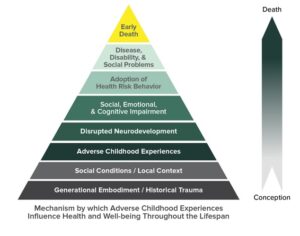









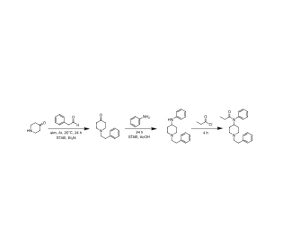
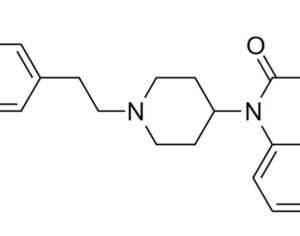




































































































































































































































































































































































































































































































































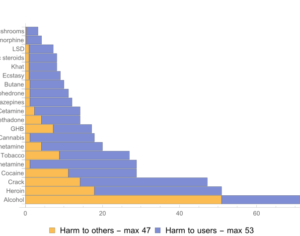































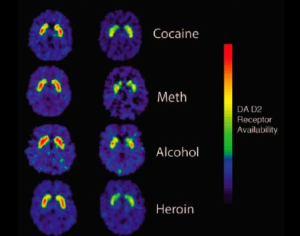











































Recent Comments