Drug made from cannabis plant gets backing from FDA staff
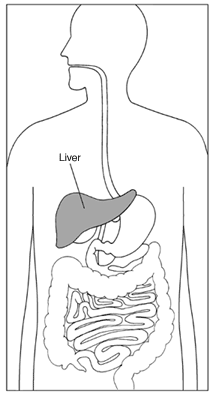
The treatment’s maker, U.K. company GW Pharmaceuticals Plc, provided “substantial evidence” of the drug’s effectiveness, FDA staff said in a report released Tuesday. The medication would treat seizures associated with two rare forms of epilepsy that typically affect children, according to the report. …the medication appears to have an increased risk of liver injury…
“The risk-benefit profile established by the data in the application appears to support approval of cannabidiol,” FDA staff wrote. The FDA has approved a few drugs made from synthetic cannabinoids, including Insys Therapeutics Inc.’s Syndros for loss of appetite in people with AIDS and nausea caused by chemotherapy. Insys is developing a cannabidiol oral solution for a severe type of epileptic seizure known as infantile spasms, and childhood epilepsy defined by staring spells where the child isn’t aware or responsive.
Links to Further Reading : Insys Therapeutics, Inc.
1) Jeff Sessions wages war on natural weed, as he grants maker of fentanyl a monopoly on synthetic THC
2) Feds arrest[ed] 6 former Insys execs for allegedly bribing doctors
3) Indicted Insys executives face October 2018 trial
4) Big pharma trying to corner the medical marijuana market
Links to Further Reading : GW Pharmaceuticals, plc
1) The Fifteen Ounce Pound : Big Pharma’s plan to patent pot
Links to Further Reading : Monsanta & Bayer
1) How Monsanto & Bayer are trying to take over the cannabis [and kratom?] industry
(The Justice Department Is Going to Let Bayer Buy Monsanto. Here’s Why It Matters)
Original Article (Bloomberg):
Drug Made From Cannabis Plant Gets Backing From FDA Staff
Artwork Fair Use: Public Domain