Former head of psychiatry products at FDA joins psychedelic drug developer…
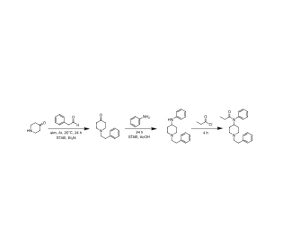
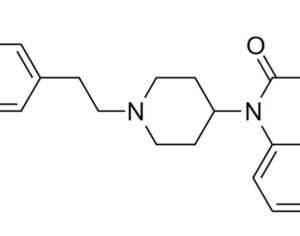
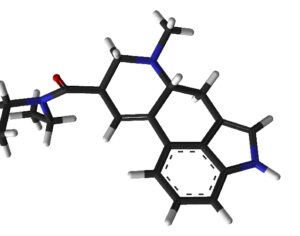
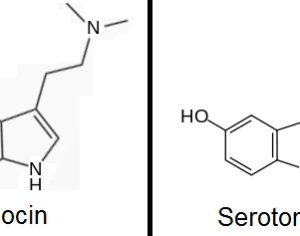
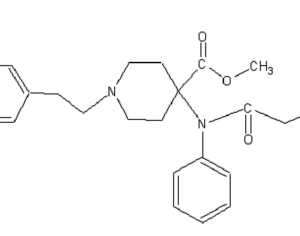
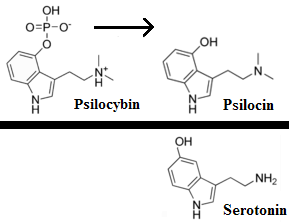
…the company is also developing novel molecules based on other psychedelic compounds. The company has filed 12 provisional patent applications for novel psychedelic molecules… Thomas Laughren, a medical doctor who served as the director of the U.S. Food and Drug Administration’s division of psychiatry products for nearly three decades…
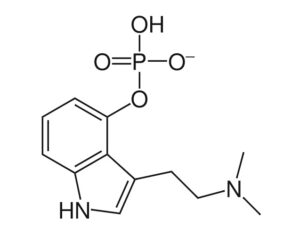
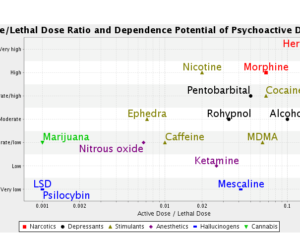
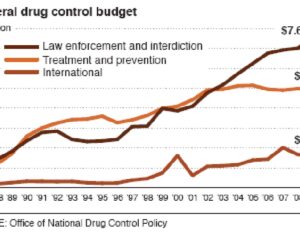
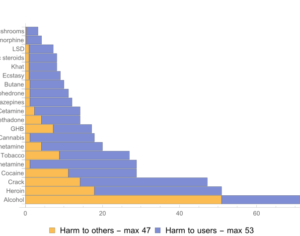
…left his post at the FDA in 2012 and has run his own consulting company to help psychiatric pharmaceutical drugmakers gain FDA approval… [claims] the future of psychedelic medicine is in new drugs that are based on classic hallucinogens like LSD and psilocybin, but are modified to shorten the trip, or remove it altogether. A psychedelic experience can last six to eight hours, which means psychedelic-assisted therapy requires doctors and therapists to work with patients for extended periods.
Original Article (Forbes):
Former head of psychiatry products at FDA joins psychedelic drug developer Cybin
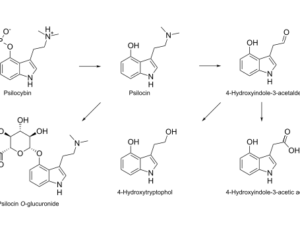
Artwork Fair Use: Saurabh R. Patil













































































































































































































































































































































































































































































































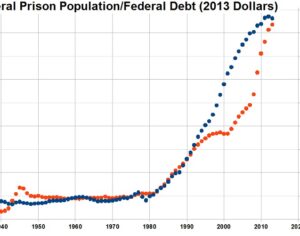
















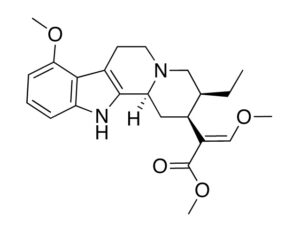






































































































Recent Comments