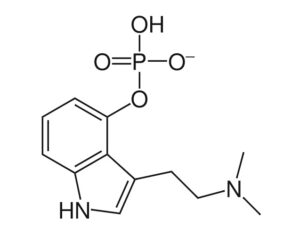
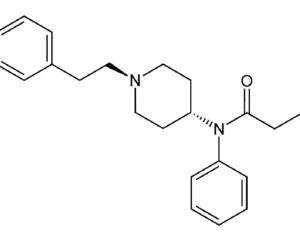
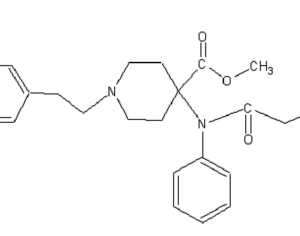
…[esketamine] touted a new antidepressant as a solution for veterans
“This was supposed to be a big game-changer,” said Dr. Erick Turner, a psychiatrist at Oregon Health and Science University. But the data on the drug (esketamine) was “nothing to write home about,” he added.
Johnson & Johnson, which owns Janssen, submitted five studies in its application to the FDA, only two of which were positive. One of those was a maintenance-of-effect study, which historically has not counted toward the two positive studies the FDA typically wants to see for approval. In particular, experts have questioned whether there is enough data to show the medication is effective in patients age 65 and over, given that many VA patients are in that population. “[The studies] are not robust. They’re not strong results. You pull one thread and the whole thing unravels,” said Turner.
Original Article (Stat):
…touted a new antidepressant as a solution for veterans. Only 15 have been treated
Artwork Fair Use: John Phelan







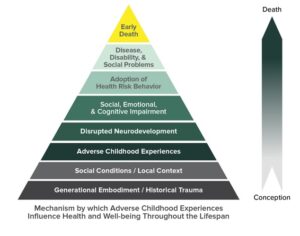























































































































































































































































































































































































































































































































































































































Recent Comments