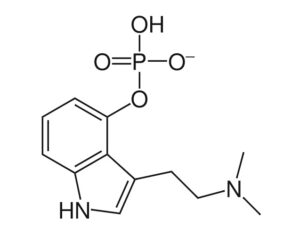
FDA slams clinical hold on tiny psychedelics player’s shot at bringing LSD-assisted therapy to more patients
…facing a holdup at the FDA in bringing its LSD program to more patients… didn’t dive into what issues the agency flagged before it made its decision.
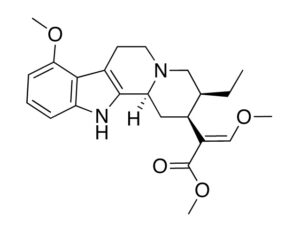
Not a whole lot is known about the New York biotech except that Barrow, formerly interim CEO and chief development officer, stepped into the full-time CEO role about a week ago, just in time to catch the bad news. His promotion came as the company’s former chairman Perry Dellelce stepped down from his role. According to its website, the company is working on creating “experiential therapies” to address neurological conditions, including its LSD-assisted therapy program for anxiety, dubbed “Project Lucy.”
Original Article (Endpts News):
FDA slams clinical hold on tiny psychedelics player’s shot at bringing LSD-assisted therapy to more patients
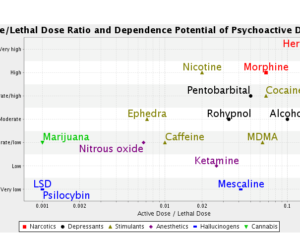
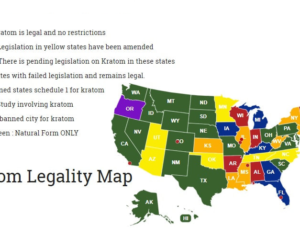
Artwork Fair Use: Anonume

























































































































































































































































































































































































































































































































































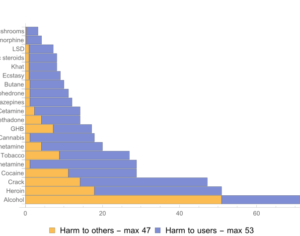































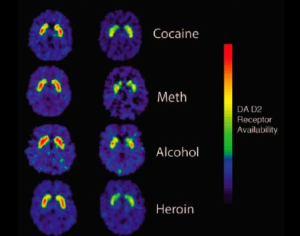











































Recent Comments