The FDA wants to stop Americans from using a popular but unapproved [by them] opioid alternative
Scott Gottlieb, the FDA’s commissioner, called for external studies on the substance, as well. “I encourage you to conduct the research that will help us better understand kratom’s risk and benefit profile, so that well-studied and potentially beneficial products can be considered,” he said. (There was no clarification on how anyone in the US should carry out said studies if they can’t get any legal imports of kratom, though.)
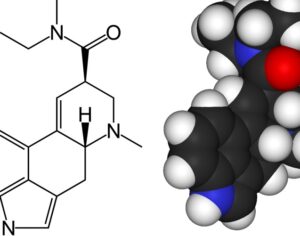
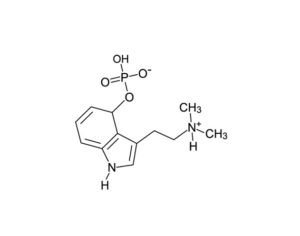
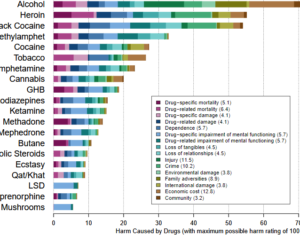
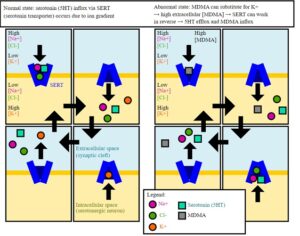
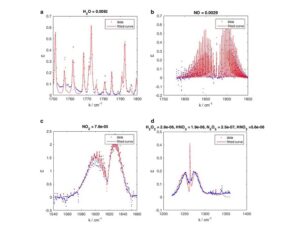
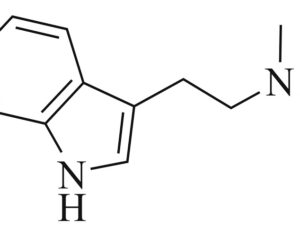
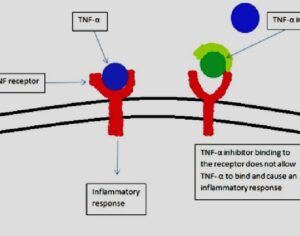
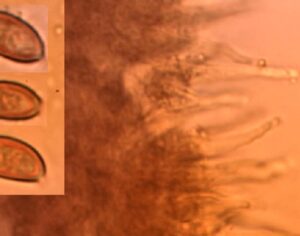
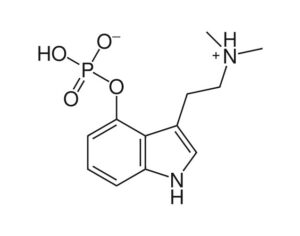
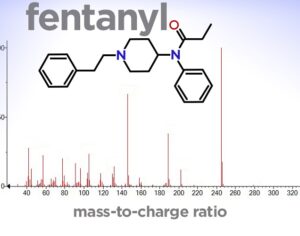
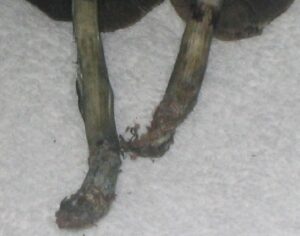
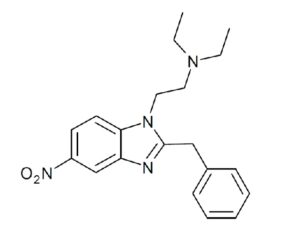
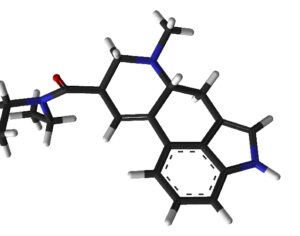
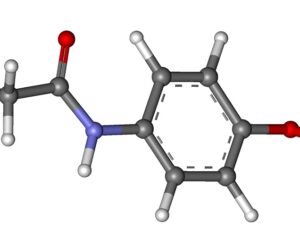
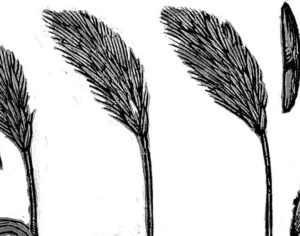
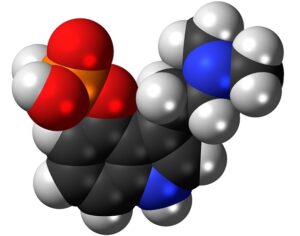
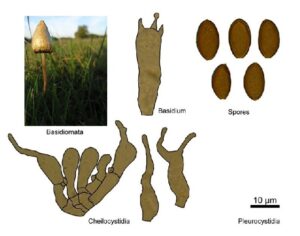
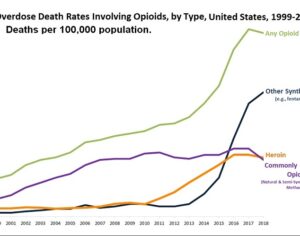
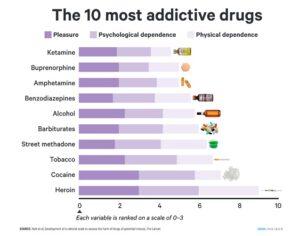
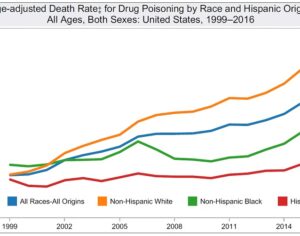
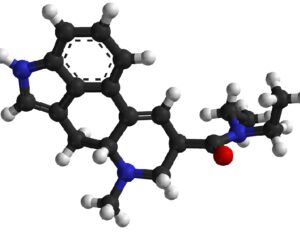
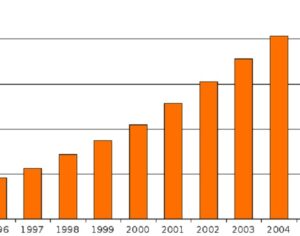
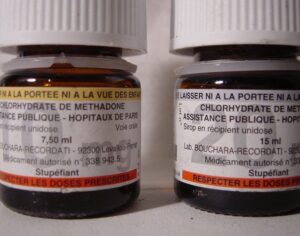
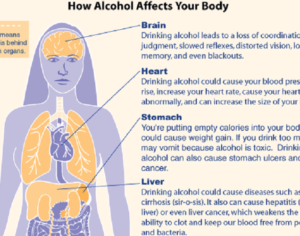
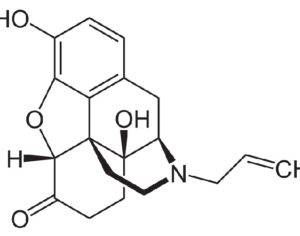
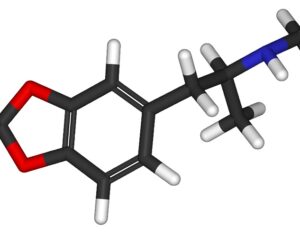
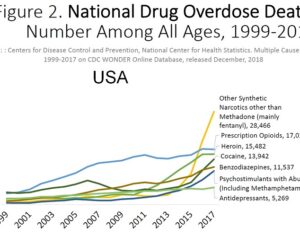
Kratom is a plant-based alternative treatment used by many as a painkiller, and by some as a way to get off opioids. Because it’s a supplement, it’s not regulated in the US. But the country’s Food and Drug Agency just issued a broad public health warning against the stuff, using terms as strong as it has when referencing deadly drugs like heroin. “I understand that there’s a lot of interest in the possibility for kratom to be used as a potential therapy for a range of disorders. But the FDA has a science-based obligation that supersedes popular trends and relies on evidence,” said Scott Gottlieb, the FDA’s commissioner, in a Nov. 14 statement on the agency’s “advisory about deadly risks associated with kratom.”
Original Article (QZ):
The FDA wants to stop Americans from using a popular but unapproved opioid alternative
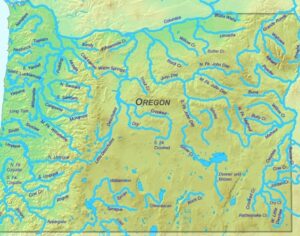
Artwork Fair Use: M.O. Stevens