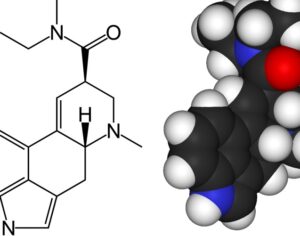
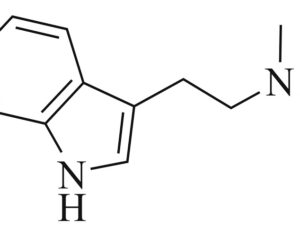
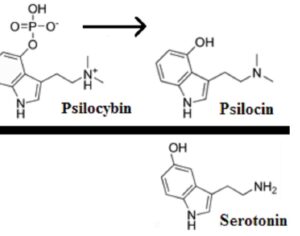
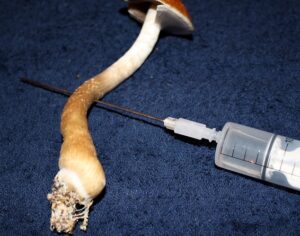
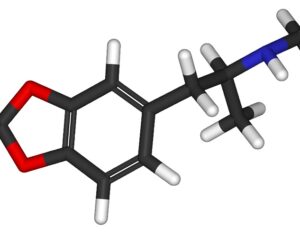
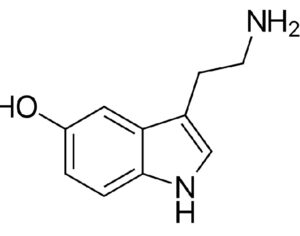
…cancer patients in federal court argument pushing DEA to allow psilocybin access
“What about under the Right to Try law, though?” asked Judge Ikuta. “Is there a pathway where they could apply under the Right to Try Act?” No, the lawyer for DEA replied. “As the agency indicated in its letter, there’s no procedure available under the Right to Try Act, because the Right to Try Act does not provide the agency any authority to waive the requirements of the Controlled Substances Act.”
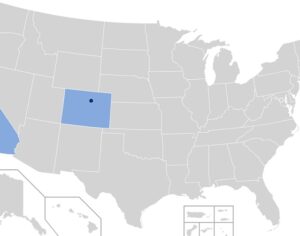
Congress and 41 U.S. states have adopted right-to-try (RTT) laws, which allow patients with terminal conditions to try investigational medications that have not been approved for general use. But in the case before the Ninth Circuit [plaintiffs] say the U.S. Drug Enforcement Administration (DEA) is standing in their way… “The average time, Phase 1 to FDA approval, is seven to 10 years,” Gonick told judges, “and Congress and 41 states determined that was just too long for some patients suffering life-threatening illnesses.”
Original Article (Marijuana Moment):
Washington officials join cancer patients in federal court argument pushing DEA to allow psilocybin access
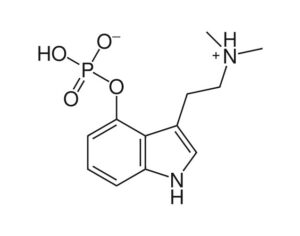
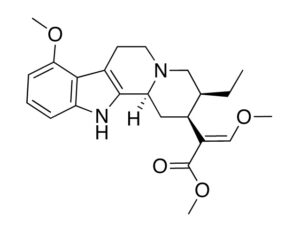
Artwork Fair Use: Dickelbers












































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































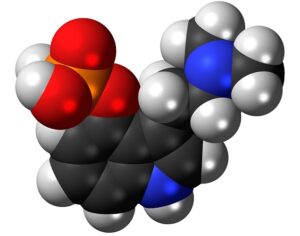



















































































































Recent Comments