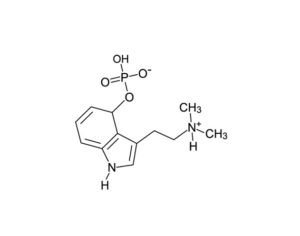
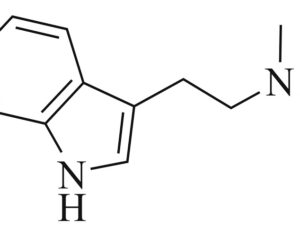
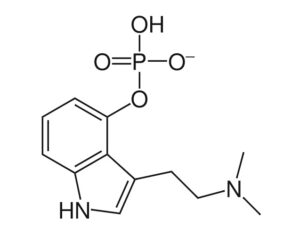
Restrictions on psilocybin ‘magic mushrooms’ are easing as research ramps up
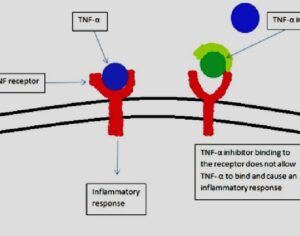
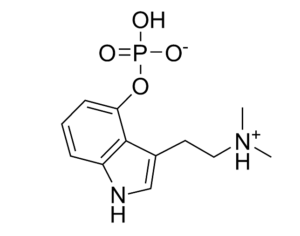
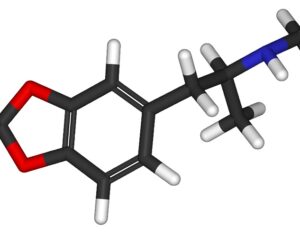
Following the 1971 United Nations Convention on Psychotropic Substances, psilocybin was classed in the U.S. as a Schedule I substance—defined as having “no currently accepted medical use and a high potential for abuse.” Psilocybin production was limited, and a host of administrative and financial burdens effectively ended study for decades. “It’s the worst censorship of research in history,” says David Nutt, a neuropsychopharmacologist at Imperial College London.
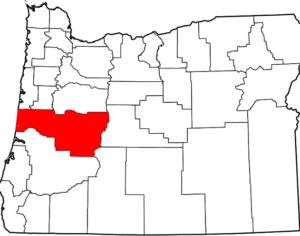
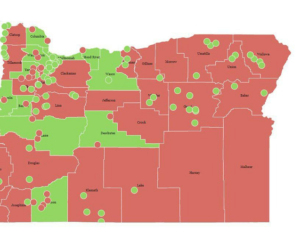
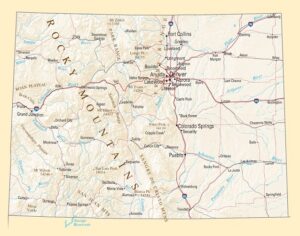
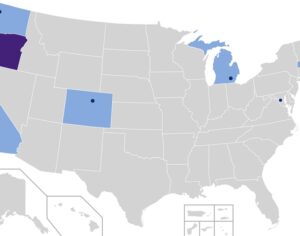
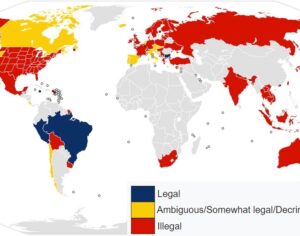
In 2019 Denver voters passed a ballot measure that prohibits using city money to prosecute people for magic mushroom–related offenses. City councils soon took similar steps in Oakland and Santa Cruz in California and in Ann Arbor, Mich. In November 2020 voters in Washington, D.C., passed a ballot measure making natural psychedelics one of law enforcement’s lowest priorities. Cities and counties in Michigan, Massachusetts, California and Washington State have followed suit. As part of Oregon’s legislation, the state health authority created a scientific advisory board to recommend regulations for psilocybin service centers, such as designating mushroom species and preparations to use and production standards to follow. These centers, which can apply for licenses starting next January, will not claim to treat depression but will aim to improve general well-being. Regardless of local decriminalization, U.S. researchers must still abide by federal Schedule I regulations. The International Therapeutic Psilocybin Rescheduling Initiative, a coalition of research and advocacy organizations, aims to get the World Health Organization to conduct a review of the relevant evidence for reclassifying the drug. “It’s inconceivable the WHO could now say psilocybin doesn’t have medical value. It can work where other drugs have not,” Nutt says.
Original Article (Scientific American):
Restrictions on psilocybin ‘magic mushrooms’ are easing as research ramps up
Artwork Fair Use: Duk































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































Recent Comments