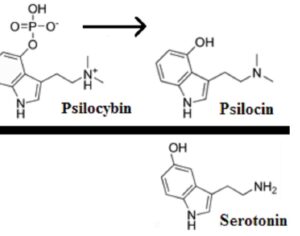
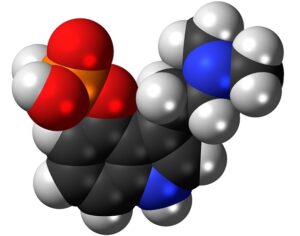
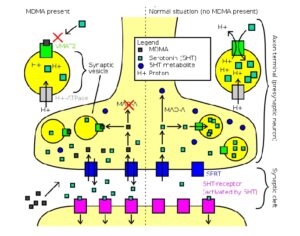
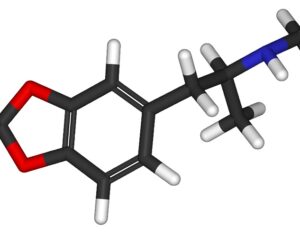
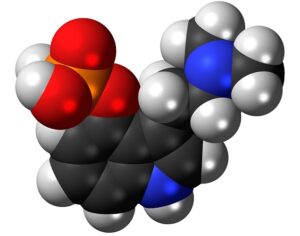
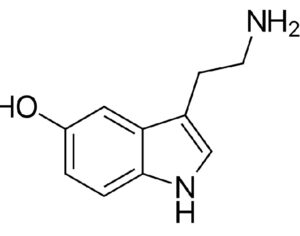
FDA weighs new application to approve MDMA as first-ever psychedelic medicine for PTSD
The MAPS Public Benefit Corporation (MAPS PBC) announced on Tuesday [12/12/23] that it submitted the new drug application (NDA) to FDA, requesting an expedited review given that the agency previously designated the psychedelic as a breakthrough therapy.
If the NDA is ultimately approved, the Drug Enforcement Administration (DEA) would then need to reschedule MDMA accordingly. It would become the first psychedelic in history to be approved as a pharmaceutical, to be administered in tandem with psychotherapy and other supportive services.
Original Article (Marijuana Moment):
FDA weighs new application to approve MDMA as first-ever psychedelic medicine for PTSD
Artwork Fair Use: Melissa McMasters































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































Recent Comments