No, the DEA did not reschedule the CBD compound
There is a certain level of confusion reverberating throughout the cannabis community right now over the DEA’s recent decision to make Epidiolex, the cannabis-based epilepsy drug created by GW Pharmaceuticals, a Schedule V controlled substance. Some folks are convinced that since this FDA-approved medication is pure cannabidiol (CBD) that all CBD products fall into the same category.
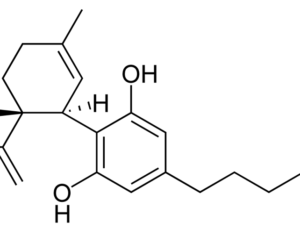
Sadly, this is not true. The federal government still considers all other cannabis-derived CBD products to be a violation of federal law. The DEA’s official statement in the Federal Register… indicates “this order places FDA-approved drugs that contain CBD derived from cannabis and no more than 0.1 percent tetrahydrocannabinols in schedule V.” What this means is the only CBD medicine considered to have, well, actual medicinal function as far as Uncle Sam is concerned is Epidiolex. The rest of it is still wrapped up in the federal marijuana ban.
The treatment’s maker, U.K. company GW Pharmaceuticals Plc, provided “substantial evidence” of the drug’s [Epidiolex] effectiveness, FDA staff said in a report. The medication would treat seizures associated with two rare forms of epilepsy that typically affect children, according to the report… the medication appears to have an increased risk of liver injury.
Original Article (Forbes):
No, the DEA did not reschedule the CBD compound
Artwork Fair Use: Hendrike