Patent… psychedelic therapeutics: a page from the cannabis playbook?
As increasing numbers of U.S. states have legalized both medical and adult-use cannabis, another set of Schedule 1 controlled substances – psychedelics – has begun to emerge from the shadows.
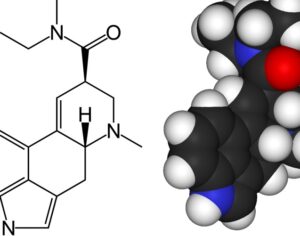
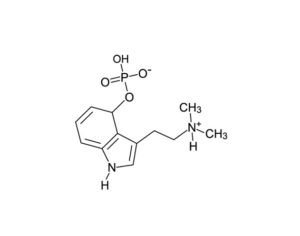
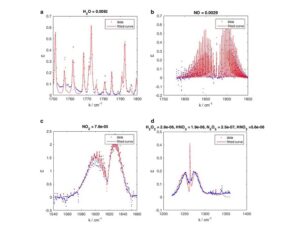
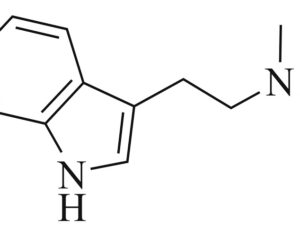
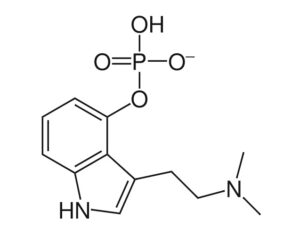
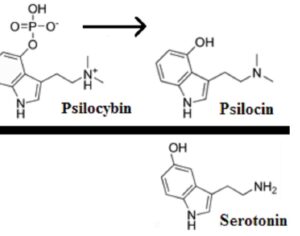
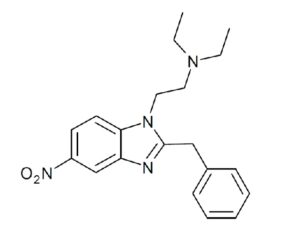
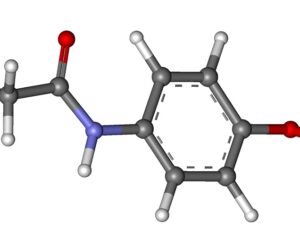
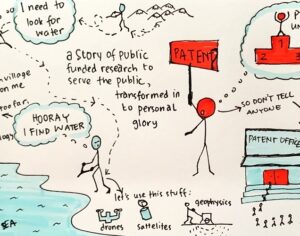
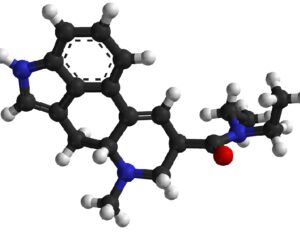
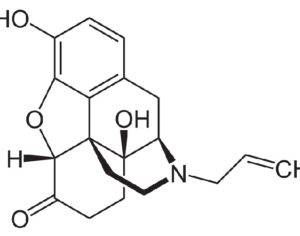
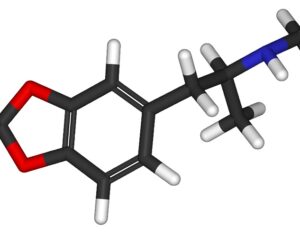
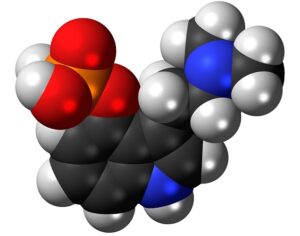
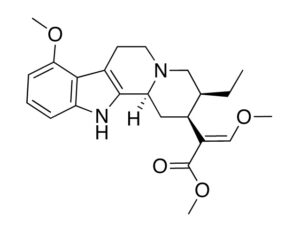
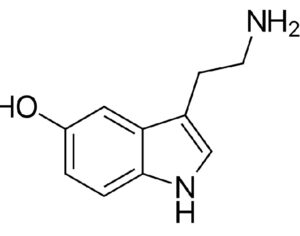
As was the case for cannabis, biomedical research into psychedelics ground nearly to a halt following the Schedule 1 designation. More than 50 years later and long after the first Sandoz Ltd. patents had expired, U.S. Patent No. 10,519,175, “Preparations of Psylocybin, Different Polymorphic Forms, Intermediates, Formulations and Their Use” was issued to Compass Pathways, Ltd., on December 31, 2019. Compass Pathways describes themselves as a mental health care company with a focus on psilocybin therapy for treatment-resistant depression. Compass Pathways’ psilocybin therapy received Breakthrough Therapy designation from the FDA in 2018. Breakthrough Therapy designation provides expedited development and review of drug candidates. The legal wrangling over U.S. Patent No. 10,519,175 (the ‘175 patent) began shortly after issuance.
Original Article (Mondaq):
Patent protection of psychedelic therapeutics: a page from the cannabis playbook?
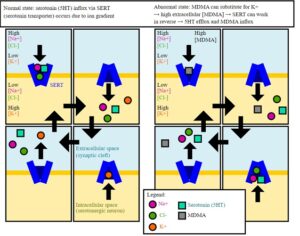
Artwork Fair Use: Public domain






























































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































Recent Comments