Federal court hears case challenging DEA decision to deny psilocybin rescheduling petition without consulting health officials
Matt Zorn, one of the attorneys representing Dr. Sunil Aggarwal, accused DEA at oral argument of wanting “to control the practice of medicine.”
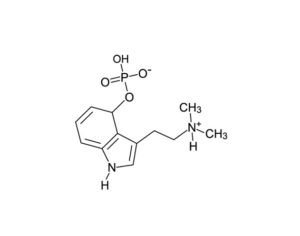
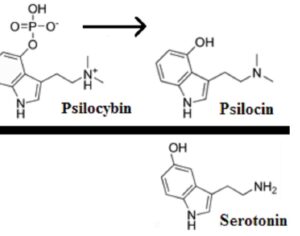
The current case, filed in the U.S. Court of Appeals for the Ninth Circuit, stems from Aggarwal’s response to last year’s ruling. In February 2022, the doctor filed a formal petition with DEA to reschedule psilocybin from Schedule I to Schedule II under the federal Controlled Substances Act (CSA). The government insists that Aggarwal’s rescheduling petition “did not address the five elements required to show that a substance has a currently accepted medical use, let alone attempt to satisfy them,” according to a filing in May that details the five-part test, which was established by DEA in 1992: Whether the substance’s chemistry is known and reproducible, Whether there are adequate safety studies, Whether there are adequate and well-controlled studies proving efficacy; Whether the substance is accepted by qualified experts; and Whether the scientific evidence is widely available. Aggarwal and his lawyers maintain that the five-part test is unlawful. During oral argument, Zorn also rejected the idea that the petition failed to address the five elements. A lengthy review paper included with the petition, he said, “meets all of the evidence of the five-part test,” but DEA simply didn’t evaluate it.
Original Article (Marijuana Moment):
Federal court hears case challenging DEA decision to deny psilocybin rescheduling petition without consulting health officials
Artwork Fair Use: Avjoska




















































































































































































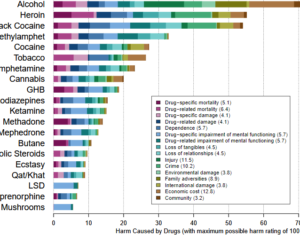






























































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































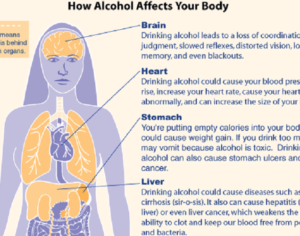




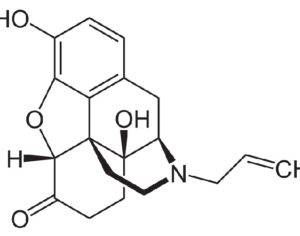






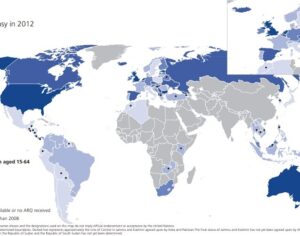
































































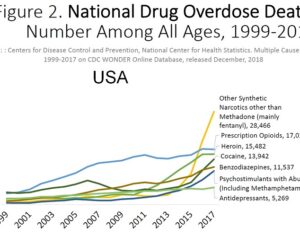



































































































Recent Comments