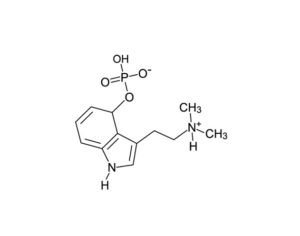
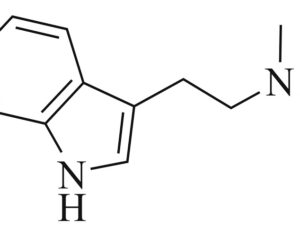
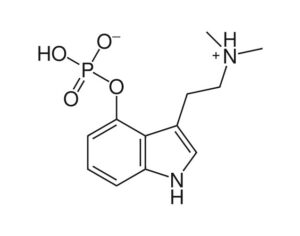
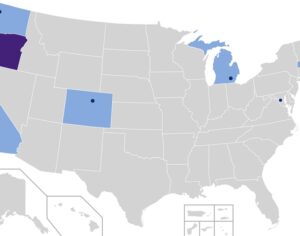
Arizona bill… psilocybin…
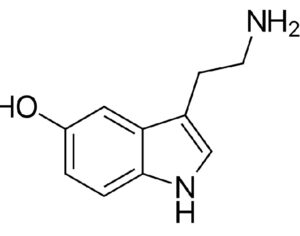
The research grants would be appropriated for phase I, II and III clinical trials that are “capable of being approved by the United States Food and Drug Administration (FDA) to evaluate the effects of whole mushroom psilocybin” for the designated conditions… to evaluate the effects of whole mushroom psilocybin on treating any of the following: post-traumatic stress disorder (PTSD), symptoms associated with long covid… depression, anxiety disorders, symptoms associated with end-of-life distress, obsessive compulsive disorder, substance abuse and addiction behaviors, eating disorders, chronic pain, inflammatory disorders, autoimmune disorders, seizure disorders, other degenerative disorders.
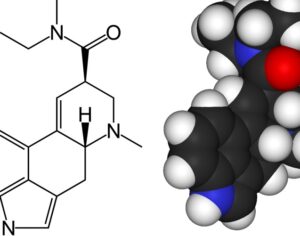
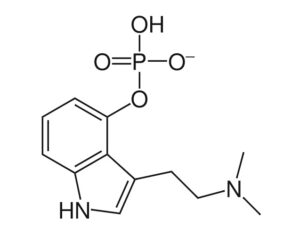
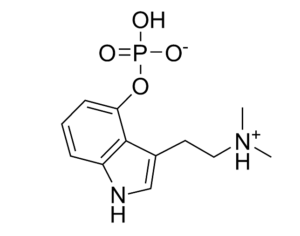
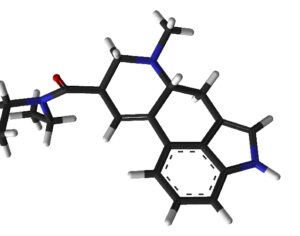
“This bill is truly groundbreaking because all of the studies showing promise with psilocybin are single molecule studies,” Sue Sisley, a researcher at the Arizona-based Scottsdale Research Institute (SRI), said. “Now finally, Arizona will lead the way to doing real world clinical trials, looking at what patients are actually ingesting in their daily lives versus an unattainable synthetic molecule. And you can see from the list of medical conditions that will be approved for study, psilocybin offers immense hope for treating a wide variety of chronic conditions including putting opioid addiction into remission (and other often intractable ailments, not responsive to conventional pharmaceuticals).”
Original Article (Marijuana Moment):
Bipartisan Arizona lawmakers file bill to promote psilocybin research with $30 million in grants & State of Arizona
Artwork Fair Use: Public domain





































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































Recent Comments