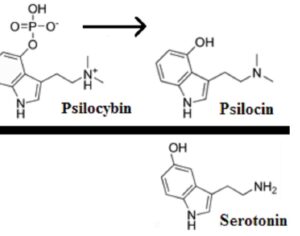
…phase 3 [synthetic COMP360] psilocybin clinical trial is… to commence
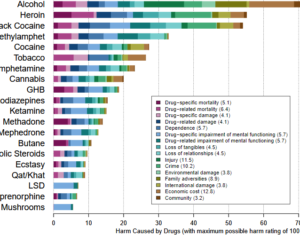
Compass… phase 2B data, which has yet to be formally published in a peer-reviewed journal… with five patients (6.3%) in the 25-mg group and six patients (8%) in the 10-mg group experiencing serious adverse events during the follow-up period….
These adverse events included suicidal ideation and suicidal behavior. The first Phase 3 trial has been dubbed Pivotal 1 and it will enroll 378 participants to compare a single 25 mg psilocybin dose to placebo. The choice of using a placebo control stands apart from the Phase 2B design which evaluated three different doses… Compass expects the first wave of data from Pivotal 1 to come late in 2024. And moves to apply to the FDA for market approval could come as soon as the following year. So if successful, this could be the clinical evidence that leads to [synthetic COMP360] psilocybin becoming a FDA-authorized medicine within three to four years.
Original Article (New Atlas):
The world’s first Phase 3 psilocybin clinical trial is about to commence
Artwork Fair Use: Ondřej Mangl































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































Recent Comments