(Federal) Right To Try act (signed) into law, opening door for medical marijuana, ecstasy (MDMA) and magic mushrooms
The signing of the bill follows a 250 to 169 vote in the House of Representatives yesterday [Tuesday, May 29th, 2018), and a unanimous vote in the Senate in August. The new law allows those with a life-threatening disease or condition to use an “eligible investigation drug” that has not received full approval from the Food and Drug Administration. The term ‘eligible investigational drug’, as defined by the new law, means a drug:
(A) for which a Phase 1 clinical trial has been completed;
(B) that has not been approved or licensed for any use under section 505 of this Act or section 351 of the Public Health Service Act;
(C)
(i) for which an application has been filed under section 505(b) of this Act or section 351(a) of the Public Health Service Act; or
(ii) that is under investigation in a clinical trial that–
(I) is intended to form the primary basis of a claim of effectiveness in support of approval or licensure under section 505 of this Act or section 351 of the Public Health Service Act; and
(II) is the subject of an active investigational new drug application under section 505(i) of this Act or section 351(a)(3) of the Public Health Service Act, as applicable; and
(D) the active development or production of which is ongoing and has not been discontinued by the manufacturer or placed on clinical hold under section 505(i)
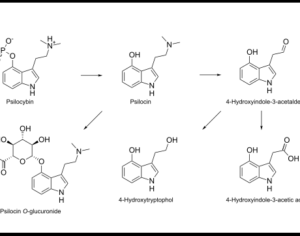
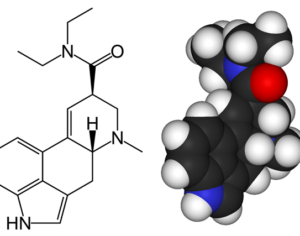
Based on this criteria, not only would marijuana apply, but so would ecstasy (MDMA) and magic mushrooms (psilocybin), which have all had Phase 1 trials completed. In the case of MDMA, they have also gone through Phase 2 trials
Original Article (The Joint Blog):
Right to try act (signed) into law, opening door for medical marijuana, ecstasy (MDMA) and magic Mushrooms
Artwork Fair Use by: ECfES