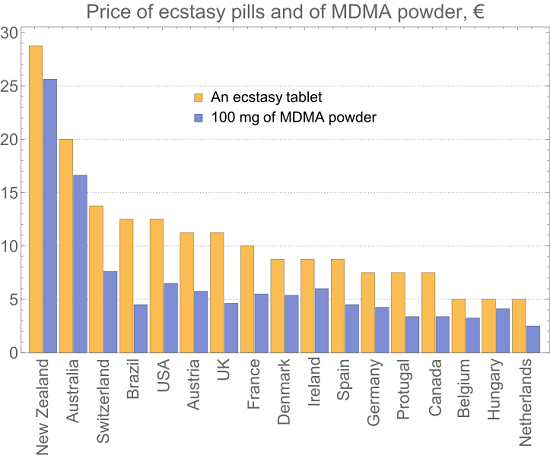
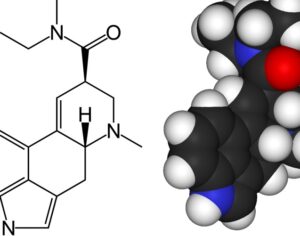
FDA gives breakthrough therapy status to MDMA-assisted psychotherapy for PTSD
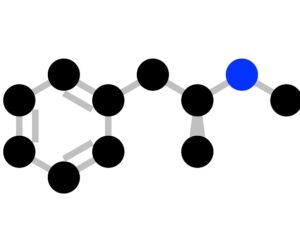
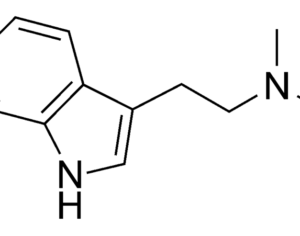
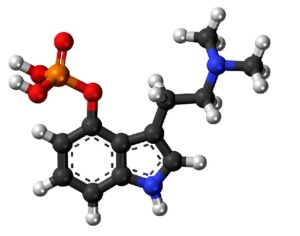
The FDA (Food and Drug Administration) has granted breakthrough therapy designation to MDMA (street name, ecstasy ; clinical name, empathy) for the treatment of posttraumatic stress disorder (PTSD).
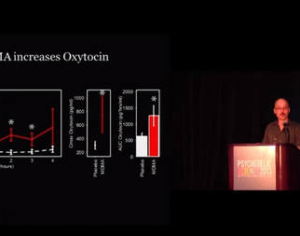
The phase 3 trials will assess the efficacy and safety of MDMA-assisted psychotherapy in 200 to 300 participants with PTSD, 18 years of age and older, at sites in the U.S., Canada, and Israel. Participants will be randomized to receive three daylong sessions of either MDMA or placebo in conjunction with psychotherapy over a 12-week treatment period, along with 12 associated 90-minute nondrug preparatory and integration sessions. The primary endpoint will be the Clinician Administered PTSD Scale, as assessed by a blinded pool of independent raters. The first phase 3 trial will begin enrolling patients in spring 2018 after the completion of an open-label lead-in training study at phase 3 sites starting this fall.
Original Article (Managed Care Mag):
FDA Gives Breakthrough Therapy Status to MDMA-Assisted Psychotherapy for PTSD
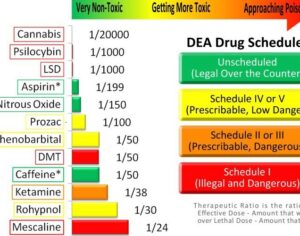
Artwork Fair Use: Melirius